Sexual dimorphism



Sexual dimorphism is the systematic difference in form between individuals of different sex in the same species. Examples include colour (specifically referred to as sexual dichromatism), size, and the presence or absence of parts of the body used in courtship displays or fights, such as ornamental feathers, horns, antlers, or tusks.
Contents |
Examples


In many species, including most mammals, the male is larger than the female.[1] In others, such as most insects, spiders, many fish, birds of prey and certain mammals such as the spotted hyena, the female is larger than the male. Other sex-specific differences include differences in colouration (sexual dichromatism), presence vs. absence of certain body parts (such as horns, antlers, tusks or display feathers), size of the eyes (some insects), possession of stings (various kinds of Hymenoptera), and different thresholds for certain behaviors (aggression, infant care, etc).
Among vertebrates, sexual dimorphism is particularly apparent in ducks and most gamefowl. This is perhaps most dramatic with species of peafowl. Male pheasants are notably larger than females and possess bright plumage, whereas females are usually brown irrespective of the particular species. In some birds, females have brighter colors than males; most of these cases are waders such as the phalaropes and painted snipes. As this is the opposite of the usual sexual dichromatism, it is termed reverse sexual dimorphism. In many predatory birds, females are larger than males, often by a considerable margin. This seems to reduce competition between members of a pair, as they have different optimal prey sizes. Some cases of sexual dimorphism in birds are so striking that males and females of the same species were originally taken to be members of entirely different species, as in the case of the Eclectus Parrot (Eclectus roratus), where the male is predominantly green with an orange beak and the female scarlet and deep blue with a black beak.
The Huia (Heteralocha acutirostris), a New Zealand bird species (now extinct), was another striking example of sexual dimorphism. The male's bill was short, sharp and stout, while the female's was long, thin and crescent-shaped. This beak dimorphism allowed mated pairs of Huia to avoid competing for the same food source, with males chiseling into and breaking apart rotting logs, while females were adept at probing into fresher wood for grubs.
Certain cases of sexual dimorphism have obvious utility beyond mate attraction. An example of this is the Blue Wildebeest (and many other biungulates). The horns of the male are much larger, allowing the male to engage in combat more effectively as he competes with other bucks for mating privileges.
An extreme example of sexual dimorphism is found in the genus Osedax of polychaete worms, which lives on whale falls. The females feed on the bones of the dead whale; the males live inside the females and do not develop past their larval stage, except to produce large amounts of sperm. In the echiuran Bonellia viridis, exposure to adult females causes larvae to develop into tiny, semi-parasitic males which are swallowed and live inside the female's genital sac. In the parasitic barnacles Sacculina, the males are tiny, free-ranging animals, whereas the females only exist as a web-like tissue inside their hosts. In the majority of scale insects, females are highly modified (eyeless and wingless, with non-functional appendages and reduced segmentation), attached permanently to their host plants, while males are rather ordinary though delicate insects, smaller and winged.
.png)
Some species of anglerfish also display extreme sexual dimorphism. Females are typical anglerfish, while males are tiny rudimentary creatures with no digestive systems. A male must find a female and fuse with her: he then lives parasitically, becoming little more than a sperm-producing body. A similar situation is found in the Zeus water bug Phoreticovelia disparata where the female has a glandular area on her back that can serve to feed a male that clings to her (note that although males can survive away from females, they generally are not free-living).[2]
Psychological and behavioral differentiation
Sex steroid-induced differentiation of adult reproductive and other behavior has been demonstrated experimentally in many animals. In some mammals, adult sex-dimorphic reproductive behavior (e.g., mounting or receptive lordosis) can be shifted to that of the other sex by supplementation or deprivation of androgens in fetal life or early infancy, even if adult levels are normal.
Evolution of sexual dimorphism
- See also: Sexual selection
In 1871 Charles Darwin advanced the theory of sexual selection, which related sexual dimorphism with sexual selection.
In many non-monogamous species, the benefit to a male's reproductive fitness of mating with multiple females is large, whereas the benefit to a female's reproductive fitness of mating with multiple males is small or non-existent.[3] In these species, there is a selection pressure for whatever traits enable a male to have more matings. The male may therefore come to have different traits from the female.
These traits could be ones that allow him to fight off other males for control of territory or a harem, such as large size or weapons;[4] or they could be traits that females, for whatever reason, prefer in mates.[5] Male-male competition poses no deep theoretical questions[6] but female choice does.
Females may choose males that appear strong and healthy, thus likely to possess "good alleles" and give rise to healthy offspring.[7] However, in some species females seem to choose males with traits that do not improve offspring survival rates, and even traits that reduce it (potentially leading to traits like the peacock's tail).[6] Two hypotheses for explaining this fact are the sexy son hypothesis and the handicap principle.
The sexy son hypothesis states that females may initially choose a trait because it improves the survival of their young, but once this preference has become widespread, females must continue to choose the trait, even if it becomes harmful. Those that do not will have sons that are unattractive to most females (since the preference is widespread) and so receive few matings.[8]
The handicap principle states that a male who survives despite possessing some sort of handicap thus proves that the rest of his genes are "good alleles." If males with "bad alleles" could not survive the handicap, females may evolve to choose males with this sort of handicap; the trait is acting as a hard-to-fake signal of fitness.[9]
Polygamy
Comparison of sexual dimorphism in birds and their mating habits shows that the time spent in search for mates, staking territories and mating competes with the demands of taking care of young. For birds and in general, it can be stated that the stronger the dimorphism in a species, the more likely is it to be polygamous and the less is the task of caring for offspring shared among the sexes. This theory is developed by R. L. Trivers' in the parental investment theory. It applies to all ecology.
Species with larger females than males
Cases where the female is larger than the male have been less widely studied,[10] and require alternate explanations. As an example, in some species females are sedentary and sparsely distributed, and so males must search for them. Vollrath and Parker argue that this difference in behaviour leads to radically different selection pressures on the two sexes, evidently favouring smaller males.[10]
Many species of birds of prey, as well as some reptiles and fish, have larger females than males.
Sexual dimorphism in humans
 |
|
 |
 |
|
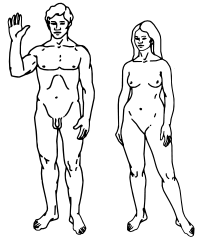
Top: Stylised illustration of humans on the Pioneer plaque, showing both male (left) and female (right). |
|
Sexual dimorphism in humans is the subject of much controversy, especially when extended beyond physical differences to mental ability and psychological gender. (For a discussion, see biology of gender, sex and intelligence, gender, and transgender.) Obvious differences between males and females include all the features related to reproductive role, notably the endocrine (hormonal) systems and their physiological and behavioural effects. Such undisputed sexual dimorphism include gonadal differentiation, internal genital differentiation, external genital differentiation, breast differentiation, muscle mass differentiation, height differentiation, and hair differentiation.
Externally, the most sexually dimorphic portions of the body are the chest, the lower half of the face, and the area between the waist and the knees.[11]
The basal metabolic rate is about 6 percent higher in adolescent males than females and increases to about 10 percent higher after puberty. Females tend to convert more food into fat, while men convert more into muscle and expendable circulating energy reserves. Females (on average) are about 52 percent as strong as males in the upper body, and about 66 percent as strong in the lower.[12] Males, on average, have denser, stronger bones, tendons, and ligaments.
Males dissipate heat faster than females through their sweat glands. Females have a greater insulation and energy reserves stored in subcutaneous fat, absorbing exothermic heat less and retaining endothermic heat to a greater degree.
Males typically have larger tracheae and branching bronchi, with about 30 percent greater lung volume per body mass. They have larger hearts, 10 percent higher red blood cell count, higher hemoglobin, hence greater oxygen-carrying capacity. They also have higher circulating clotting factors (vitamin K, prothrombin and platelets). These differences lead to faster healing of wounds and higher peripheral pain tolerance.[13]
Females typically have more white blood cells (stored and circulating), more granulocytes and B and T lymphocytes. Additionally, they produce more antibodies at a faster rate than males. Hence they develop fewer infectious diseases and succumb for shorter periods.[13] Ethologists argue that females, interacting with other females and multiple offspring in social groups, have experienced such traits as a selective advantage.[14][15][16][17][18] Note that almost all examples of sexual dimorphism in humans are quantitative, and have some degree of overlap.
Some biologists theorise that a species' degree of sexual dimorphism is inversely related to the degree of paternal investment in parenting. Species with the highest sexual dimorphism, such as the pheasant, tend to be those species in which the care and raising of offspring is done only by the mother, with no involvement of the father (low degree of paternal investment).
Although there are many biologically-determined, sexually-dimorphic behaviours in other species, these have few, if any, implications for human society. However, analysis of sexually dimorphic human behavior naturally provokes controversy. One less controversial, but still hypothetical, area with considerable discussion in academic literature concerns potential evolutionary advantages associated with sexual competition (both intrasexual and intersexual) and short- and long-term sexual strategies.[19]
According to Daly and Wilson, "The sexes differ more in human beings than in monogamous mammals, but much less than in extremely polygamous mammals."[20]
D.M. Buss stated that "Males should prefer attributes in potential mates associated with reproductive value or fertility, depending on whether males in human evolutionary history have tended to seek long-term or short-term mating partners.[21] Specifically, if males in our evolutionary past have tended to seek short-term mating partners, selection should have favoured male preferences for females in their early 20s who show cues positively correlated with fertility. If males in our evolutionary past tended to seek long-term mating partners, selection should have favoured preferences for females in their mid-teens who show cues indicative of reproductive value. Evolutionary theorists differ on which of these they judge to be most likely." However, there has not yet been a study to prove that males chose who they actually end up mating with or, rather that it is the females who chose the males.[22]
Sexual dimorphism in birds
Sexual dimorphism in birds can be manifested in size or plumage differences between the sexes. Sexual size dimorphism varies among taxa with males typically being larger, though this is not always the case i.e. birds of prey.[23] Plumage dimorphism, in the form of ornamentation or coloration, also varies, though males are typically the more ornamented or brightly colored sex.[24] Such differences have been attributed to the unequal reproductive contributions of the sexes.[25] In some species, the male’s contribution to reproduction ends at copulation, while in other species the male becomes the main caregiver. Plumage polymorphisms have evolved to reflect these differences and other measures of reproductive fitness, such as body condition[26] or survival.[27] The male phenotype sends signals to females who then choose the ‘fittest’ available male.
Sexual dimorphism is a product of both genetics and environmental factors. An example of sexual polymorphism determined by environmental conditions exists in the house finch. House finch males can be classified into three categories during breeding season: black breeders, brown breeders, and brown auxiliaries[26]. These differences arise in response to the bird’s body condition: if they are healthy they will produce more androgens thus becoming black breeders, while less healthy birds produce less androgens and become brown auxiliaries[26]. The reproductive success of the male is thus determined by his success during each year’s non-breeding season, causing reproductive success to vary with each year’s environmental conditions.
Sexual dimorphism is maintained by the counteracting pressures of natural selection and sexual selection. For example, sexual dimorphism in coloration increases the vulnerability of bird species to predation by European sparrowhawks in Denmark.[28] Presumably, increased sexual dimorphism means males are brighter and more conspicuous, leading to increased predation.[28] Moreover, the production of more exaggerated ornaments in males may come at the cost of suppressed immune function.[26] So long as the reproductive benefits of the trait due to sexual selection are greater than the costs imposed by natural selection, then the trait will propagate throughout the population. Reproductive benefits arise in the form of a larger number of offspring, while natural selection imposes costs in the form of reduced survival. This means that even if the trait causes males to die earlier, the trait is still beneficial so long as males with the trait produce more offspring than males lacking the trait.
Such differences in form and reproductive roles often cause differences in behavior. As previously stated, males and females often have different roles in reproduction. The courtship and mating behavior of males and females are regulated largely by hormones throughout a bird’s lifetime.[29] Activational hormones occur during puberty and adulthood and serve to ‘activate’ certain behaviors when appropriate, such as territoriality during breeding season.[29] Organizational hormones occur only during a critical period early in development, either just before or just after hatching in most birds, and determine patterns of behavior for the rest of the bird’s life.[29] Such behavioral differences can cause disproportionate sensitivities to anthropogenic pressures.[30] Females of the whinchat in Switzerland breed in intensely managed grasslands.[30] Earlier harvesting of the grasses during the breeding season lead to more female deaths.[30] Populations of many birds are often male-skewed and when sexual differences in behavior increase this ratio, populations decline at a more rapid rate.[30]
Consequently, sexual dimorphism has important ramifications for conservation. However, sexual dimorphism is not only found in birds and is thus important to the conservation of many animals. Such differences in form and behavior can lead to sexual segregation, defined as sex differences in space and resource use.[31] Most sexual segregation research has been done on ungulates,[31] but such research extends to bats[32], kangaroos[33], and birds[34]. Sex-specific conservation plans have even been suggested for species with pronounced sexual segregation.[32]
See also
|
|
References
Notes
- ↑ Lindenfors P, Gittleman JL & Jones KE, Sexual size dimorphism in mammals, in: Fairbairn DJ, Blanckenhorn WU & Szekely T (eds) Sex, size and gender roles: evolutionary studies of sexual size dimorphism (Oxford: Oxford University Press, 2007), pages 19-26
- ↑ Arnqvist, Göran , Therésa M. Jones, Mark A. Elgar (2003)Reversal of sex roles in nuptial feeding. Nature 424:387 [1]
- ↑ Futuyma (2005) Evolution (1st Edition). Sunderland, Massachusetts: Sinauer Associates. Page 330.
- ↑ Futuyma (2005) Evolution (1st Edition). Sunderland, Massachusetts: Sinauer Associates. Page 331.
- ↑ Futuyma (2005) Evolution (1st Edition). Sunderland, Massachusetts: Sinauer Associates. Page 332.
- ↑ 6.0 6.1 Ridley M (2004). Evolution (3rd Edition). Malden, Massachusetts: Blackwell Publishing. Page 328.
- ↑ Futuyma (2005) Evolution (1st Edition). Sunderland, Massachusetts: Sinauer Associates. Page 335.
- ↑ Ridley M (2004). Evolution (3rd Edition). Malden, Massachusetts: Blackwell Publishing. Page 330.
- ↑ Ridley M (2004). Evolution (3rd Edition). Malden, Massachusetts: Blackwell Publishing. Page 332.
- ↑ 10.0 10.1 Vollrath F, Parker GA (1992) Sexual dimorphism and distorted sex ratios in spiders. Nature 360(6400): 156-159 (doi:10.1038/360156a0)
- ↑ Gray 1918, Nowell 1926, Green 2000, et al.
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/8477683
- ↑ 13.0 13.1 Glucksman, A. (1981) Sexual Dimorphism in Human and Mammalian Biology and Pathology (Academic Press, 1981), pp. 66-75
- ↑ J Durden-Smith and D Desimone, Sex and the Brain, (New York: Arbor House, 1983).
- ↑ ES Gersh and I Gersh, Biology of Women, (Baltimore: University Park Press, 1981).
- ↑ J Stein (editor), Internal Medicine, 2nd edition., (Boston: Little, Brown and Company, 1987).
- ↑ M McLaughlin and T Shryer, 'Men vs Women: The New Debate Over Sex Differences', U.S. News & World Report 8 August (1988): pp. 50-58.
- ↑ BS McEwen, 'Neural Gonadal Steroid Action', Science 211 (1981): 1303–1311.
- ↑ (David M Buss, 2007)
- ↑ Martin Daly and Margo Wilson, 'Evolutionary Psychology and Marital Conflict', in Sex, Power, Conflict: Evolutionary and Feminist Perspectives, edited by DM Buss and Neil M Malamuth, (Oxford University Press, 1996), p. 13.
- ↑ (Buss 1987; Symons 1979; Williams 1975)
- ↑ DM Buss, 'Evolutionary theory', in Personality: Critical Concepts, edited by Cary L Cooper and Lawrence A Pervin, (Routledge, 1998), p. 422.
- ↑ Andersson, Malte B.(1994). Sexual selection. Princeton University Press. p. 269. ISBN 0-6910-0057-3. Google Book Search. Retrieved on November 04, 2009.
- ↑ McGraw, K. J.; Hill, G. E.; Stradi, R.; & Parker, R. S. (2002). "The effect of dietary carotenoid access on sexual dichromatism and plumage pigment composition in the American goldfinch". Comparative Biochemistry and Physiology Part B-Biochemistry and Molecular Biology 131(2), 261-269.
- ↑ Owens, I. P. F. & Hartley, I. R. (1998). "Sexual dimorphism in birds: why are there so many different forms of dimorphism?". Proceedings of the Royal Society Part B-Biological Sciences 265(1394), 397-407.
- ↑ 26.0 26.1 26.2 26.3 Lindsay, W. R. M.; Webster, M. S.; Varian, C. W.; & Schwabl, H. (2009). "Plumage colur acquisition and behaviour are associated with androgens in a phenotypically plastic bird". Animal Behaviour 77(6), 1525-1532.
- ↑ Petrie, M. (1994). "Improved growth and survival of offspring of peacocks with more elaborate trains". Nature 371(6498), 598-599.
- ↑ 28.0 28.1 Moller, A. P. & Nielsen, J. T. (2006). "Prey vulnerability in relation to sexual coloration of prey". Behavioral Ecology and Sociobiology 60(2), 227-233.
- ↑ 29.0 29.1 29.2 Adkins-Regan, E. (2007). "Hormones and the development of sex differences in behavior". Journal of Ornithology 148(Supplement 1), S17-S26.
- ↑ 30.0 30.1 30.2 30.3 Grubler, M. U.; Schuler, H.; Muller, M.; Spaar, R.; Horch, P.; & Naef-Daenzer, P. (2008). "Female biased mortality caused by anthropogenic nest loss contributes to population decline and adult sex ratio of a meadow bird". Biological Conservation 141(12), 3040-3049.
- ↑ 31.0 31.1 Main, M. B. (2008). "Reconciling competing ecological explanations for sexual segregation in ungulates". Ecology 89(3), 693-704.
- ↑ 32.0 32.1 Safi, K.; Konig, B.; & Kerth, G. (2007). "Sex differences in population genetics, home range size and habitat use of the parti-colored bat (Vespertilio murinus, Linnaeus 1758) in Switzerland and their consequences for conservation". Biological Conservation 137(1), 28-36.
- ↑ Coulson, G.; MacFarlane, A. M.; Parsons, S. E.; & Cutter, J. (2006). "Evolution of sexual segregation in mammalian herbivores: kangaroos as marsupial models". Australian Journal of Zoology 54(3), 217-224.
- ↑ Gonzalez-Solis, J.; Croxall, J. P.; & Wood, A. G. (2000). "Sexual dimorphism and sexual segregation in foraging strategies of northern giant petrels, Macronectes halli, during incubation". Oikos 90(2), 390-398
Bibliography
- Bonduriansky, R. (2007) The evolution of condition-dependent sexual dimorphism. The American Naturalist, 169:1 pp9–19.
- Biological Journal of the Linnean Society (1999), 67: 1–18.
External links
|
||||||||||||||||||||||||||||||||||||||||||||
